Nanoparticles
Nanoparticles
A particle is defined as a small object that behaves as a whole unit with respect to its transport and properties. Coarse particles cover a range between 10,000 and 2,500 nanometers. Fine particles are sized between 2,500 and 100 nanometers. Ultrafine particles or nanoparticles are sized between 1 and 100 nanometers. Nanoparticles are a discovery of modern science and they have a very long history. Nanoparticles were used by artisans as far back as the 9th century in Mesopotamia for generating a glittering effect on the surface of pots. This lustre or glitter over pottery from the Middle Ages and Renaissance is due to a metallic film that was applied to the transparent surface of a glazing. The lustre can still be visible if the film has resisted atmospheric oxidation and other weathering. There are several methods for production of nanoparticles.
Source: https://cargocollective.com/AndrewPenn/Nano-Particles-Nano-Robotics
Source: http://news.mit.edu/2012/cancer-particle-0404
- In attrition method, a macro or micro scale particles are ground in a ball mill, a planetary ball mill, or other size reducing mechanism. The resulting particles are air classified to recover nanoparticles.
- In pyrolysis method, a vaporous precursor (liquid or gas) is forced through an orifice at high pressure and burned. The resulting solid (a version of soot) is air classified to recover oxide particles from by-product gases. Pyrolysis often results in aggregates and agglomerates rather than single primary particles.
- Inert-gas condensation is frequently used to make nanoparticles from metals with low melting points. In this process the metal is vaporized in a vacuum chamber and then super cooled with an inert gas stream. The super cooled metal vapor condenses in to nanometer-sized particles, which can be entrained in the inert gas stream and deposited on a substrate or studied in situ.
- Nanoparticles can also be formed using radiation chemistry. Radiolysis from gamma rays can create strongly active free radicals in solution. This relatively simple technique uses a minimum number of chemicals. These including water, a soluble metallic salt, a radical scavenger (often a secondary alcohol), and a surfactant (organic capping agent). Formation of nanoparticles using the radiolysis method allows for tailoring of particle size and shape by adjusting precursor concentrations and gamma dose.
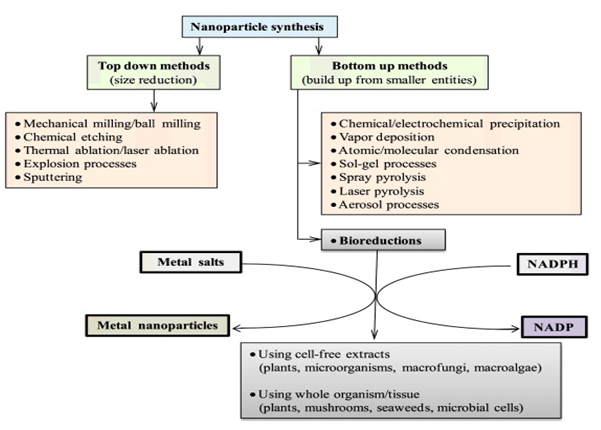
Source: https://www.researchgate.net/profile/Loutfy_Madkour/publication/323573533/figure/ fig4/AS:631590698885172@1527594365591/Various-approaches-for- making-nanoparticles-and-cofactor-dependent-bio-reduction.png
Source: https://nanoquest.files.wordpress.com/2013/08/nanoparticle.jpg
Source: http://summer.catsgarden.net/academic/uploads/2009/11/hair-nanoparticles.png