ENVIS Centre, Ministry of Environment & Forest, Govt. of India
Printed Date: Friday, August 22, 2025
Arsenic
Arsenic
Arsenic is a natural element found in soil and minerals. Arsenic compounds are used to preserve wood, as pesticides, and in some industries. Arsenic can get into air, water, and the ground from wind-blown dust. It may also get into water from runoff. Arsenic is a metalloid - a natural element that is not actually a metal but which has some of the properties of a metal. It is a natural component of the Earth’s crust, generally found in trace quantities in all rock, soil, water and air. However, concentrations may be higher in certain areas due to either natural conditions or human activities.
Arsenic Chemistry
- Several oxidation states:
- As-1 in sulphide minerals,
- As0, metal, only stable in very reduced conditions but can be reduced to As-3 in the most toxic form of arsine gas (AsH3)
- As3+, As5+ are common in oxidizing conditions and soluble at all values of Eh and pH
- Oxidation of As3+ to less toxic As5+ is slow so usually both are present in oxidized environments like mine tailings.
- Arsenic can be removed from mine water by the addition of a solution containing FeSO4.
- Fe2+ is oxidized to Fe3+ and precipitates as FEOOH
- Arsenate is strongly absorbed by FeOOH and precipitated
| WHO |
in drinking water |
10µg/l (maximum) |
| EU |
in drinking water |
50 µg/l |
| US EPA |
in drinking water |
50 µg/l |
| Arsenic contamination in Groundwater |
Argentina, Bangladesh, Chile, China, Hungary, Nepal, India, Mexico, Romania, Taiwan, Vietnam, SW USA, Myanmar |
| Arsenic Contamination from Geothermal Water |
Argentina, Dominica, Chile, France, Japan, Iceland, New Zealand, Alaska USA |
| Arsenic Contamination in Mining Effluents |
Canada, Ghana, Greece, Italy, Russia, Thailand, USA |
Toxicity of Arsenic
- AsO4-3 replaces PO4-3 and cells die
- AsO4-3 inhibits oxidative phosphorylation in the ATP energy cycle
- AsO3-3 replaces S in thiol groups and inhibits protein functions
- Absorbed by inhalation or digestion and transferred via the bloodstream to all organs producing systemic damage.
- Long term low level exposure causes hyper pigmentation (black spots on skin), followed by skin malignancy, peripheral arteriosclerosis (black foot disease)
- Lung, liver and kidney cancer develop over time.
- Acute arsenic exposure results in vomiting, abdominal pain and bloody diarrhea and death.
- Men and children are more affected than women.
- Bangladesh about 20% of wells are contaminated and an estimated 80 million people are dependent on those wells for domestic purposes and affected by arsenic poisoning.
Skin diseases caused by Arsenic toxicity
Source: https://yourhealthofima.wordpress.com/2012/06/02/arsenicosis-a-global-problem/
Source: https://diseaeseshows.com/arsenic_poison_symptom/
Environmental transport and distribution
Arsenic is emitted into the atmosphere by high-temperature processes such as coal-fired power generation plants, burning vegetation and volcanism. Natural low-temperature biomethylation and reduction to arsines also releases arsenic into the atmosphere. Arsenic is released into the atmosphere primarily as As2O3 and exists mainly adsorbed on particulate matter. These particles are dispersed by the wind and are returned to the earth by wet or dry deposition.
Arsines released from microbial sources in soils or sediments undergo oxidation in the air, reconverting the arsenic to non-volatile forms, which settle back to the ground. Dissolved forms of arsenic in the water column include arsenate, arsenite, methylarsonic acid (MMA) and dimethylarsinic acid (DMA). In well-oxygenated water and sediments, nearly all arsenic is present in the thermodynamically more stable pentavalent state (arsenate). Some arsenite and arsenate species can interchange oxidation state depending on redox potential (Eh), pH and biological processes. Some arsenic species have an affinity for clay mineral surfaces and organic matter and this can affect their environmental behavior. There is potential for arsenic release when there is fluctuation in Eh, pH, soluble arsenic concentration and sediment organic content. Weathered rock and soil may be transported by wind or water erosion. Many arsenic compounds tend to adsorb to soils, and leaching usually results in transportation over only short distances in soil.
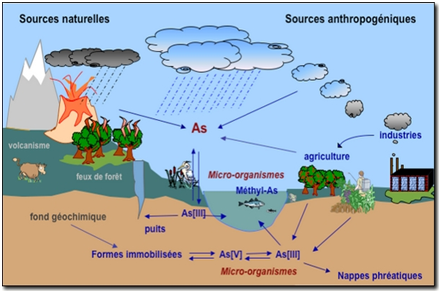
Figure 1. Cycle of Arsenic in the environment.
Source: http://coursenligne.u-strasbg.fr/pages.jsp
Figure 2. Arsenic contamination in Bangladesh.
Source: http://osmaninagor.org
Back to top of page